Proven, de-risked, and scalable solar technology to fight climate change

How Synhelion produces renewable fuels
The sun provides us with enormous amounts of energy every day – but unlocking its full potential for our needs remains a major challenge. At Synhelion, we’ve developed an innovative technology to harness this abundant energy and efficiently convert it into sustainable fuels. Besides renewable energy, we need water and a carbon source to produce renewable fuels.

The carbon source – turning biogenic waste into biogas
Synhelion uses RED-certified sustainable biogenic waste as carbon source. This cost-effective, mostly agricultural waste undergoes a natural process called anaerobic digestion, converting the biogenic waste into raw biogas. This raw biogas, a mixture of biogenic methane and CO2, serves as the ideal feedstock for our fuel production.

The energy source – turning sunlight into high-temperature process heat
To process the raw biogas into fuel, we need high-temperature process heat. We either use renewable electricity from a PV field to drive our proprietary electric gas heater, or we use our proprietary concentrated solar system (heliostats and solar receiver) to produce renewable solar process heat over 1’100°C to power the chemical conversion process.
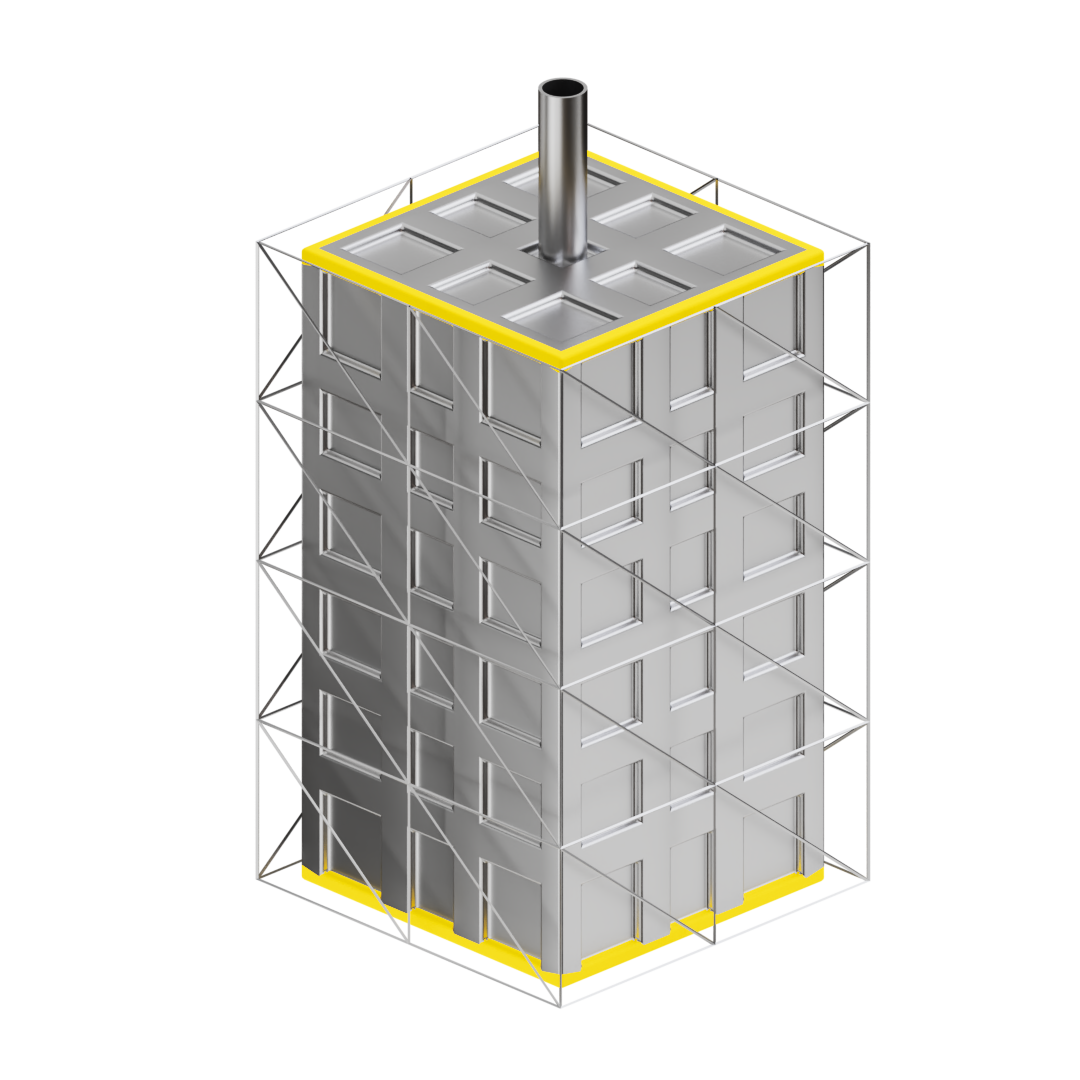
The thermal energy storage – ensuring solar fuel production 24/7
The solar energy generated during the day is stored in our proprietary thermal energy storage system, enabling round-the-clock operation of the plant. This system stores energy much cheaper than battery storage. It allows us to keep our plants running efficiently for around 8’000 hours per year, which is the industry standard for cost-effective operation.
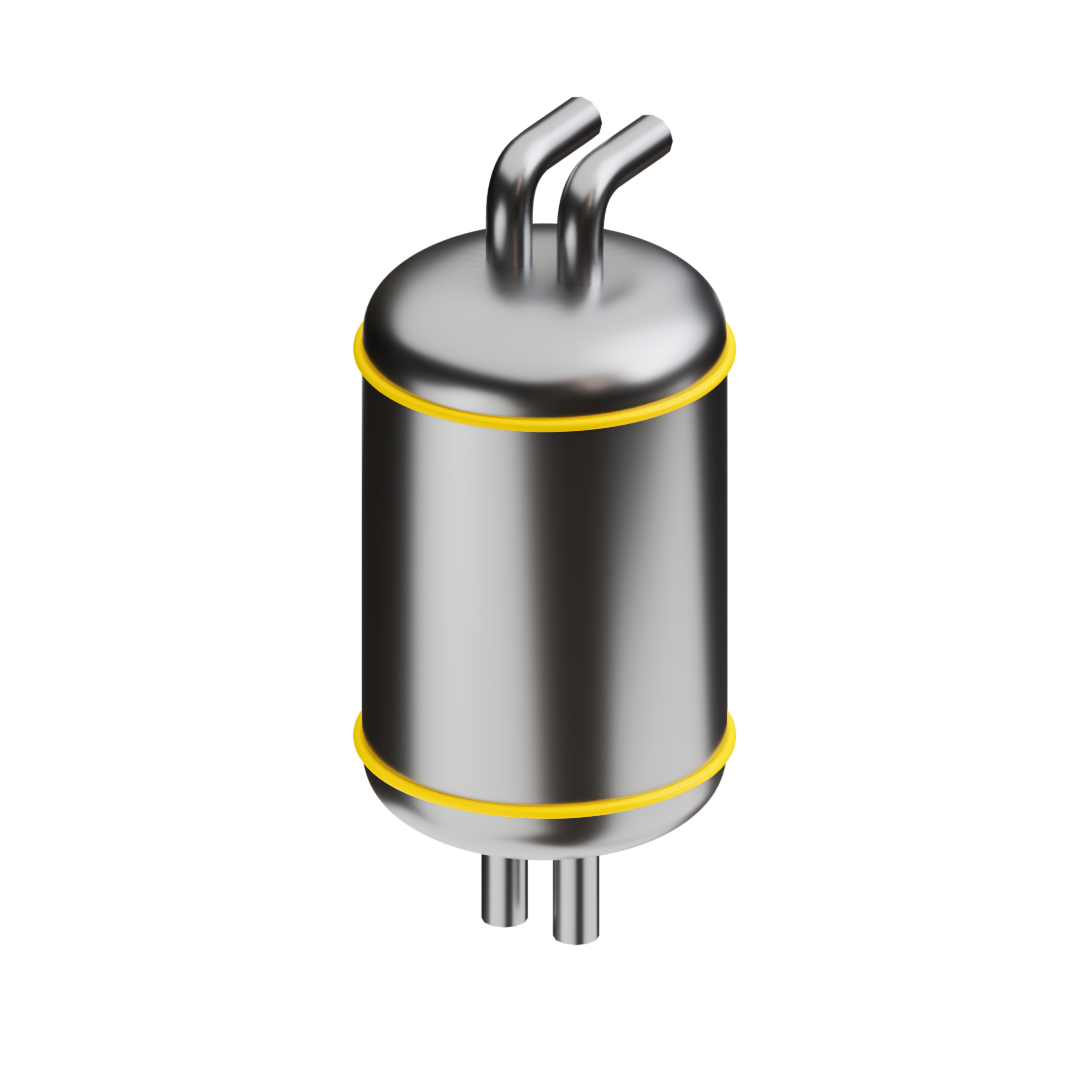
The thermochemical reactor – converting biogas into syngas
The high-temperature process heat drives our proprietary thermochemical reactor. In this reactor, the biogas and water molecules are rearranged to create syngas, a mixture of hydrogen (H2) and carbon monoxide (CO). This process stores the solar energy in the chemical bonds of syngas, making it available for later use.

The fuel synthesis – turning syngas into liquid solar fuels
Syngas is the universal key to produce liquid fuels. We use standard industrial methods to liquefy the syngas and finally yield solar drop-in fuels such as renewable jet fuel, diesel, and gasoline. These fuels are ready to be used in today’s engines and infrastructure.

The result – sustainable solar fuels, efficient and available around the clock
By combining affordable feedstocks, cheap renewable energy, and cost-effective thermal energy storage, we efficiently produce sustainable fuels day and night, helping to create a cleaner, more sustainable future for global transportation.
-
Scientific publications
Synhelion partners with top-tier research labs and conducts cutting-edge research. See below for a selection of our most important publications.
-
Zuber, M., Patriarca, M., Ackermann, S., Furler, P., Conceição, R., Gonzalez-Aguilar, J., Romero, M., Steinfeld, A., “Methane dry reforming via a ceria-based redox cycle in a concentrating solar tower”. Sustainable Energy & Fuels, 8 (2023).
-
Schäppi, R., Rutz, D., Dähler, F., Muroyama, A., Haueter, P., Lilliestam, J., Patt, A., Furler, P., Steinfeld, A., “Drop-in fuels from sunlight and air”. Nature (2021).
-
Ambrosetti, G., Good, P., “A novel approach to high temperature solar receivers with an absorbing gas as heat transfer fluid and reduced radiative losses”. Solar Energy, 183, 521–531 (2019).
-
Furler, P., Scheffe, J., Marxer, D., Gorbar, M., Bonk, A., Vogt, U., Steinfeld, A., “Thermochemical CO2 splitting via redox cycling of ceria reticulated foam structures with dual-scale porosities”. Physical Chemistry Chemical Physics 16, 10503–10511 (2014).
-
Marxer, D., Furler, P., Scheffe, J., Geerlings, H., Falter, C., Batteiger, V., Sizmann, A., Steinfeld, A., “Demonstration of the entire production chain to renewable kerosene via solar thermochemical splitting of H2O and CO2”. Energy & Fuels (2015).
-
Furler, P., Scheffe, J. R., Steinfeld, A., “Syngas production by simultaneous splitting of H2O and CO2 via ceria redox reactions in a high-temperature solar reactor”. Energy & Environmental Science 5, 6098–6103 (2012).
-
Marxer, D., Furler, P., Takacs, M., Steinfeld, A., “Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency”. Energy & Environmental Science 10, 1142–1149 (2017).
-
Ackermann, S., Scheffe, J., Steinfeld, A., “Diffusion of oxygen in ceria at elevated temperatures and its application to H2O/CO2-splitting thermochemical redox cycles”. The Journal of Physical Chemistry, 118, 5216–5225 (2014).
-
Geissbühler, L., “Thermocline thermal energy storage: advances and applications to CSP, compressed air energy storage, and solar fuels”. Diss ETH No. 24555, (2017).
-
Geissbühler, L., Kolman, M., Zanganeh, G., Haselbacher, A., Steinfeld, A., “Analysis of industrial-scale high-temperature combined sensible/latent thermal energy storage”. Applied Thermal Engineering 101, 657–668 (2016).
-
Geissbühler, L., Mathur, A., Mularczyk, A., Haselbacher, A., “An assessment of thermocline-control methods for packed-bed thermal-energy storage in CSP plants, Part 1: Method descriptions”, Solar Energy 178, 341–350 (2019).
-
Dähler, F., Wild, M., Schäppi, R., Haueter, P., Cooper, T., Good, P., Larrea, C., Schmitz, M., Furler, P., Steinfeld, A., “Optical design and experimental characterization of a solar concentrating dish system for fuel production via thermochemical redox cycles”. Solar Energy, 170, 568–575 (2018).
-
Chueh, W., Falter, F., Abbott, M., Scipio, D., Furler, P., Haile, S., Steinfeld, A., “High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria”. Science, 330, 1797-1801, 2010.
-
Ackermann, S., Scheffe, J., Duss, J., Steinfeld, A., “Morphological characterization and effective thermal conductivity of dual-scale reticulated porous structures”. Materials, 7, 7173-7195 (2014).
-
Moretti, C., Patil, V., Falter, C., Geissbühler, L., Patt, A., Steinfeld, A., “Technical, economic and environmental analysis of solar thermochemical production of drop-in fuels”. Science of the Total Environment, 901, 166005 (2023).
-




